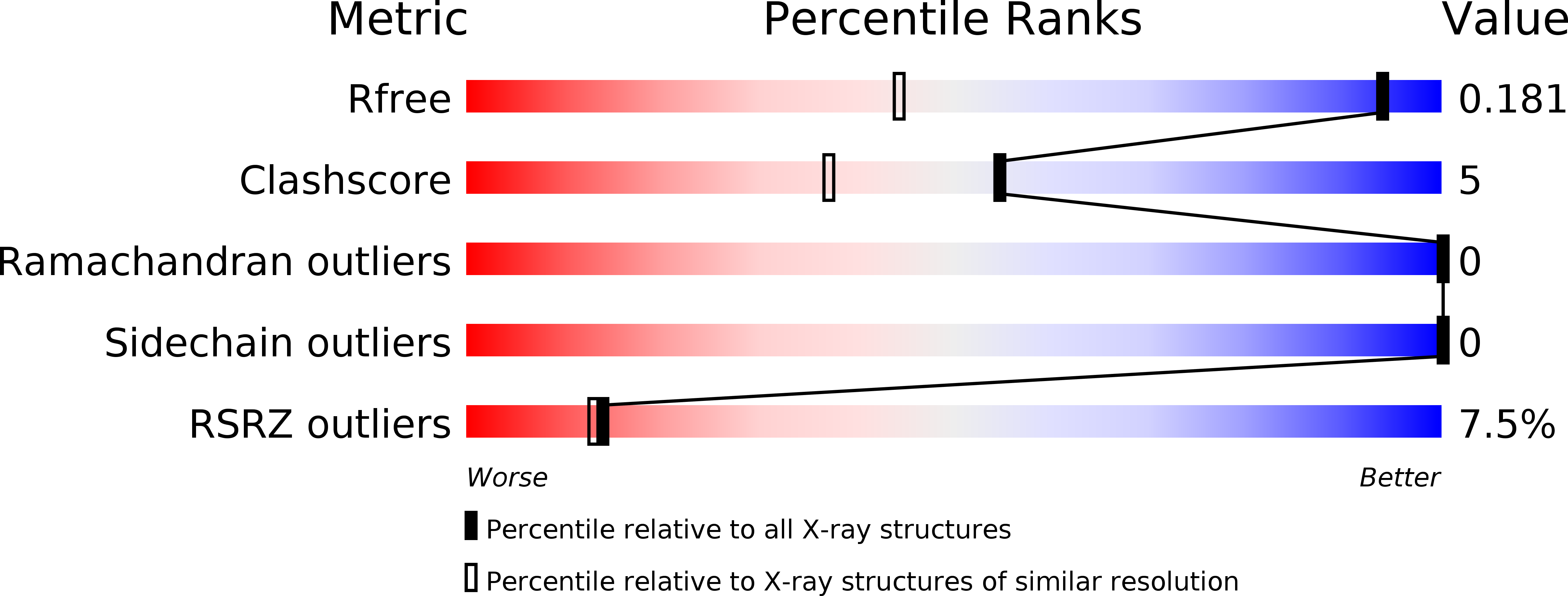
Structural States of Hdm2 and HdmX: X-ray Elucidation of Adaptations and Binding Interactions for Different Chemical Compound Classes.
Kallen, J., Izaac, A., Chau, S., Wirth, E., Schoepfer, J., Mah, R., Schlapbach, A., Stutz, S., Vaupel, A., Guagnano, V., Masuya, K., Stachyra, T.M., Salem, B., Chene, P., Gessier, F., Holzer, P., Furet, P.(2019) ChemMedChem 14: 1305-1314
- PubMed: 31066983
- DOI: https://doi.org/10.1002/cmdc.201900201
- Primary Citation of Related Structures:
6Q96, 6Q9H, 6Q9L, 6Q9O, 6Q9Q, 6Q9S, 6Q9U, 6Q9W, 6Q9Y - PubMed Abstract:
Hdm2 (human MDM2, human double minute 2 homologue) counteracts p53 function by direct binding to p53 and by ubiquitin-dependent p53 protein degradation. Activation of p53 by inhibitors of the p53-Hdm2 interaction is being pursued as a therapeutic strategy in p53 wild-type cancers. In addition, HdmX (human MDMX, human MDM4) was also identified as an important therapeutic target to efficiently reactivate p53, and it is likely that dual inhibition of Hdm2 and HdmX is beneficial. Herein we report four new X-ray structures for Hdm2 and five new X-ray structures for HdmX complexes, involving different classes of synthetic compounds (including the worldwide highest resolutions for Hdm2 and HdmX, at 1.13 and 1.20 Å, respectively). We also reveal the key additive 18-crown-ether, which we discovered to enable HdmX crystallization and show its stabilization of various Lys residues. In addition, we report the previously unpublished details of X-ray structure determinations for eight further Hdm2 complexes, including the clinical trial compounds NVP-CGM097 and NVP-HDM201. An analysis of all compound binding modes reveals new and deepened insight into the possible adaptations and structural states of Hdm2 (e.g., flip of F55, flip of Y67, reorientation of H96) and HdmX (e.g., flip of H55, dimer induction), enabling key binding interactions for different compound classes. To facilitate comparisons, we used the same numbering for Hdm2 (as in Q00987) and HdmX (as in O15151, but minus 1). Taken together, these structural insights should prove useful for the design and optimization of further selective and/or dual Hdm2/HdmX inhibitors.
Organizational Affiliation:
Chemical Biology & Therapeutics, Novartis Institutes for BioMedical Research, Novartis Campus, 4002, Basel, Switzerland.